We have seen that a different geometric arrangement of bonds in space can produce distinct molecules, stereoisomers, despite the same bonding structure. Even within the same molecule, however, different spatial arrangements are available to a molecule due to rotations about bonds. Different spatial arrangements due to rotations about a bonds are called conformations.
Table of contents:
Conformational analysis of ethane
A conformational analysis is a study of the energetics of different spatial arrangements of atoms relative to rotations about bonds. Conformational analyses are assisted greatly by a representation of molecules in a manner different to skeletal structures. A representation of a molecule in which the atoms and bonds are viewed along the axix about which rotation occurs is called a Newman projection (Figure 1).
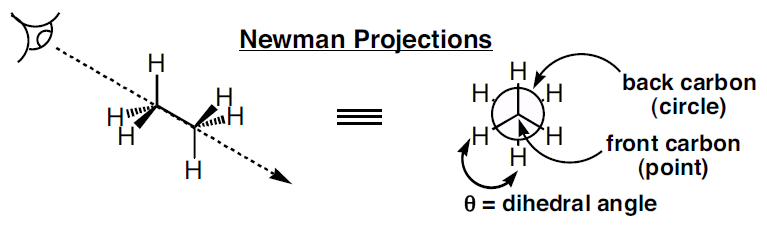
Figure 1: Newman projection of ethane
In a Newman projection, the molecule is viewed along an axis containing two atoms bonded to each other and the bond between them, about which the molecule can rotate. In a Newman projection, the "substituents" of each atom composing the bond, be they hydrogens or functional groups, can then be viewed both in front of and behind the carbon-carbon bond. Specifically, one can observe the angle between a substituent on the front atom and a substituent on the back atom in the Newman projection, which is called the dihedral angle or torsion angle.
In ethane specifically, we can imagine two possible "extreme" conformations. In one case, the dihedral angle is 0° and the hydrogens on the first carbon line up with or eclipse the hydrogens on the second carbon. When the dihedral angle is 0° and the hydrogens line up perfectly, ethane has adopted the eclipsed conformation (Figure 2). The other extreme occurs when the hydrogens on the first carbon are as far away as possible from those on the second carbon; this occurs at a dihedral angle of 60° and is called the staggered conformation (Figure 2).
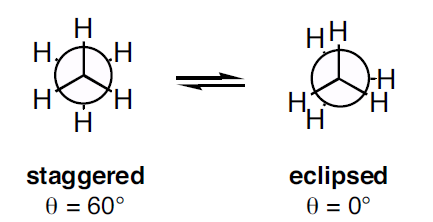
Figure 2: Conformations of ethane - staggered and eclipsed
The staggered conformation of ethane is a more stable, lower energy conformation than the eclipsed conformation because the eclipsed conformation involves unfavorable interactions between hydrogen atoms. Specifically, the negatively charged electrons in the bonds repel each other most when the bonds line up. Thus, ethane spends most of its time in the more stable staggered conformation.
Conformational analysis of butane
In butane, two of the substituents, one on each carbon atom being viewed, is a methyl group. Methyl groups are much larger than hydrogen atoms. Thus, when eclipsed conformations occurs in butane, the interactions are especially unfavorable. There are four possible "extreme" conformations of butane: 1) Fully eclipsed, when the methyl groups eclipse each other; 2) Gauche, when the methyl groups are staggered but next to each other; 3) Eclipsed, when the methyl groups eclipse hydrogen atoms; and 4) Anti, when the methyl groups are staggered and as far away from each other as possible (Figure 3).
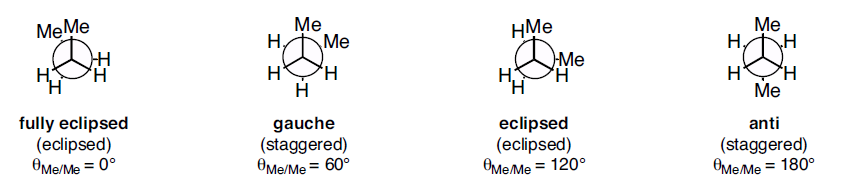
Figure 3: Conformations of butane: fully eclipsed, gauche, eclipsed, and anti
The fully eclipsed conformation is clearly the highest in energy and least favorable since the largest groups are interacting directly with each other. As the molecule rotates, it adopts the relatively stable gauche conformation. As it continues to rotate, it encounters less favorable eclipsed conformation in which a methyl group eclipses a hydrogen. As rotation continues, the molecule comes to the anti conformation, which is the most stable since the substituents are staggered and the methyl groups are as far away from each other as possible. An energy diagram is a graph which represents the energy of a molecule as a function of some changing parameter. An energy diagram can be made as a function of dihedral angle for butane (Figure 4).
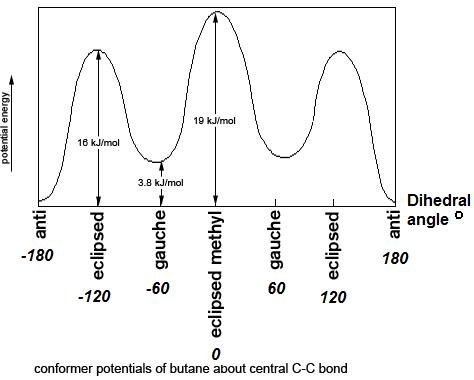
Figure 4: Energy diagram for conformations of butane as a function of dihedral angle
Clearly, the anti and gauche conformations are significantly more stable than the eclipsed and fully eclipsed conformations. Butane spends most of its time in the anti and gauche conformations.
There is one final, very important point. At room temperature, approximately 84 kJ/mol of thermal energy is available to molecules. Thus, if the barrier to a rotation is less than 84 kJ/mol, the molecule will rotate. In ethane and butane, the barriers to rotation are significantly less than 84 kJ/mol. Therefore, even though the eclipsed conformations are unfavorable, the molecules are able to adopt them. In reality, since these conformations are not as stable, the molecules will quickly pass through them at room temperature and return a staggered conformation. Molecules are constantly converting between different staggered conformations all the time, quickly passing through eclipsed conformations in between. Thus, in alkanes, no single "true" conformation exists all the time; the molecule instead constantly converts between conformations, spending more time in those that are more stable. This constant conversion lies in stark contract to alkenes, which adopt the cis- or trans- (E- or Z-) conformations and retain them at room temperature; they do not interconvert because the barrier to rotation is too high.
Note
This website is the result of my experience tutoring organic chemistry. It is intended to educate students in the basic principles of organic chemistry and present the material in a clear and easy to understand manner. It aims to provide greater clarity and detail and teach differently than organic chemistry textbooks.
| Previous topic: Stereochemistry |
5. Conformational analaysis of alkanes
Back to index |
Next topic: Cyclic systems - cyclohexane |